Hurting from a boo-boo? Blame Mrgprb2!
Our fun aside for this week is Cre-Lox recombination! And no, this has nothing to do with smoked salmon.
Suppose you’d like to target a cell population based on a protein it expresses (think Mrgpr2). Maybe you want to make those cells susceptible to a toxin so you can wipe them out of your mouse. Maybe you just want to fluorescently label them. The Cre-Lox system handles this problem by exploiting the same promoter as your gene of interest. When a mouse is bred to have a Cre-protein that is controlled by the same promoter as your gene of interest, Cre will be expressed whenever your gene of interest is expressed. What makes Cre special is that it chops out DNA between sequences of loxP sites. This DNA will only be cut out if there are two loxP sites flanking the region (the fancy scientists call this a “floxed” gene). Usually you order mice with the region you want cut out already floxed, but if you hate yourself you can genetically engineer this on your own.
In the picture below, a mouse is bred to have Cre under the same promoter of a floxed gene (blue) and a reporter gene (green). When some cell in this mouse tries expressing the floxed gene, Cre will be expressed and cut this gene out. This cell and all of its descendants will never be able to produce the floxed protein again. It is important that this process is localized only to cells that have tried expressing the floxed gene.
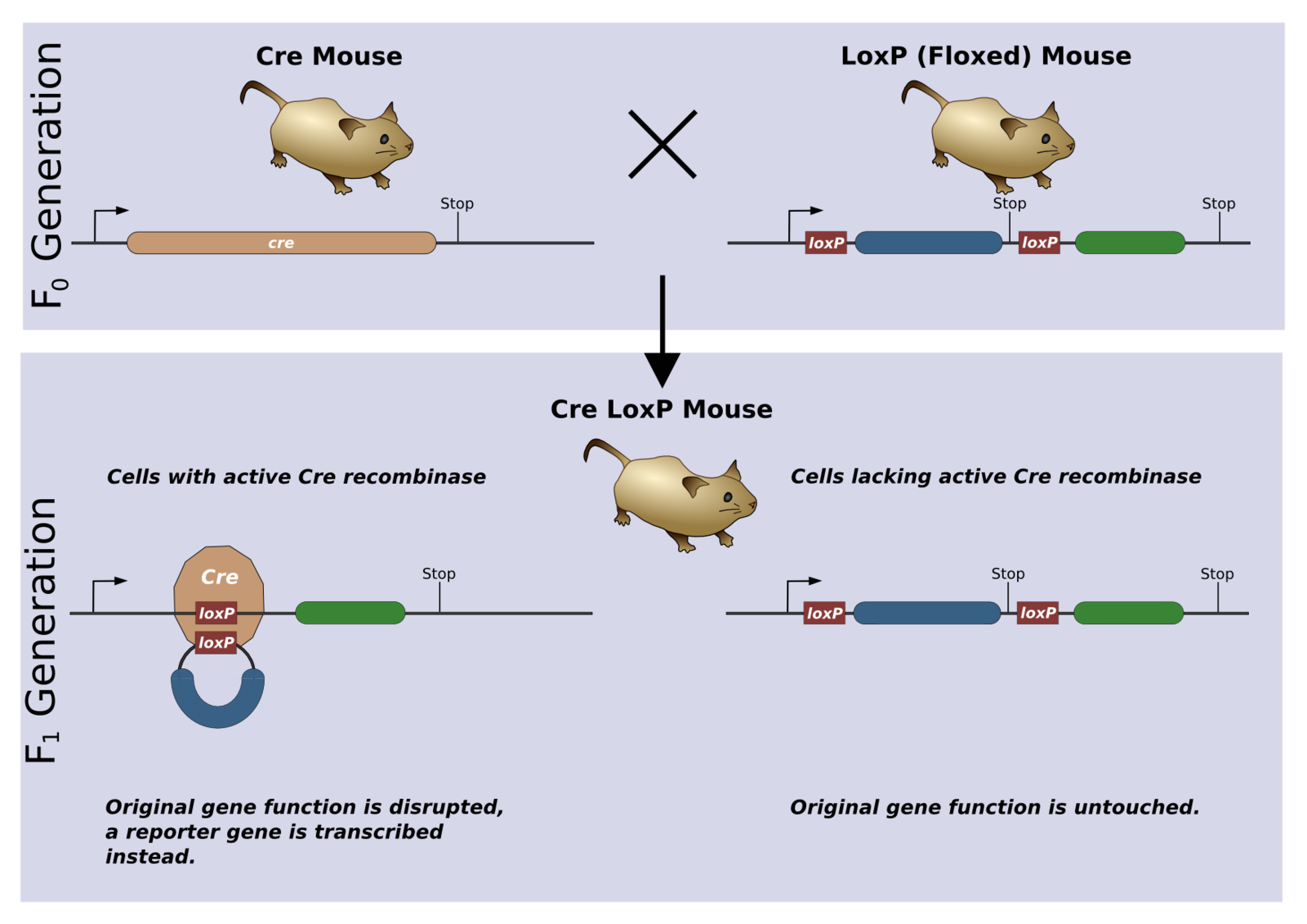
After the floxed gene (blue) has been excised, your reporter gene (green) is now expressed. This gene could encode a fluorescent protein that allows you to visualize cells in which the floxed gene was removed. Or, the reporter protein could bind a toxin that selectively kills cells of interest. Once you inject the toxin into your mouse, any cell that had its floxed gene removed will be wiped out, and the rest will be unharmed.